usp class vi compliant
Fitting body constructed of durable polysulfone. Enflo products are USP Class VI FDA ROHS REACH and Conflict Materials compliant.
But Dursan is one of the first CVD silicon coatings to achieve USP Class VI compliance.
. Valves restrict flow when fitting halves are disconnected. 7 USP Class VI materials EPDM silicone fluorocarbon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600. Compliance to USP Class VI is often requested by users in the biopharmaceutical and medical industries.
What is ADI-Free BSE-Free TSE-Free. Class VI testing is aimed to certify that there are no harmful reactions or long-term bodily effects caused by chemicals that leach out of plastic materials. United States Pharmacopeia USP 26 NF21 2003 Class VI.
SIMONA PP-H USP Class VI sheet is ideal for applications requiring biocompatibility testing standards defined by ISO 109931. Master Bond systems are very versatile and can be used for both disposable and reusable medical devices. Typical applications for our FDA NSF 51 USDA materials are disposable medical.
There are six classes VI being the most rigorous. Coatings like Dursan prevent protein binding and carryover in analytical systems and prevent system cross contamination while improving corrosion resistance and durability. Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds.
Dursan can be applied to more durable base materials like stainless steel to. Three chapters are applicable to elastomers plastics and polymeric materials. Compounds made without animal-derived ingredients BSETSE concerns.
ADI-free certifies that the raw materials used in production of the elastomer contain no Animal Derived Ingredients ADI. Sil 714002 USP class VI Silicone 1 70 Yes transl. Suitability under USP Class VI is typically a base requirement for medical device manufacturers.
Pharmacopoeia Class VI judges the suitability of plastic material intended for use as containers or accessories for parenteral preparations. USP Class VI Compliant Materials for Medical and Pharmaceutical Products Meets USP Class VI requirements for use in medical and pharmaceutical applications. When production of the elastomer contain no ADI with respect to source manufacture and treatment they cannot.
There are plenty of silicone products and other medical grade plastics that are USP Class VI compliant. Specifically USP publishes test instructions for the plastics polymers and elastomers that are used in medical devices and surgical equipment. Body and seal materials are made of USP Class VI-compliant and BSETSE-free materials.
RoHS a European Union Directive restricts the use of certain substances but manufacturers also need to know whether all the ingredients in a medical silicone are made of compliant materials. These CPC quick-disconnect fittings feature an automatic shutoff valve which closes off the flow path at disconnection protecting valuable media while eliminating the need. Moulded O-rings class 1 less than 10 furnace black These can be produced in all possible dimensions up to diameter 1400 mm internal.
Why is that important. Specially formulated for long term sealing. Table 1 shows our standard programme FDA compliant com- FDA and USP class VI compliant.
The USP Class VI compounds must be made from ingredients with clear histories of biocompatibility that meet tighter requirements for leachates. The USP defines six plastics classes from class I to class VI with class VI being the most rigorous and most frequently requested certification. The USP classification system for plastics groups them into Classes I VI defined by compliance testing and approval criteria.
USP Class VI Approved Plastic Materials. ISO 90012015 Certified QMS. Has a full range of specialty adhesives epoxies primers for polyolefins UV curables and silicones that have been fully tested to meet USP Class VI requirements.
Testing for biocompatibility of materials is mainly centered on guidelines put forth by ISO 10993 the FDA United States Regulations EU 2017745-6 European Union and the USP. Sil 714001 USP class VI Silicone 1 70 Yes transl. Pharmacopeia USP a non-profit organization whose standards inform decision-making at the US.
ENFLON is a registered trademark for. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. PPE manufactures medical and pharmaceutical grade gaskets and seals including O-rings sanitary gaskets and other high performance seals from a range of 15 USP Class VI compliant elastomers-.
Many plastics manufacturers find it advantageous to have their materials classified especially if their plastic resins are a likely candidate to be used in medical devices. Overview of USP Class VI Approved Plastic Materials. Food and Drug Administration FDA.
All these special grade products have passed this rigorous test. USP Class Testing standards are determined by the United States. Some medical silicones must meet USP Class VI FDA CFR 21 1772600 and RoHS requirements.
USP Class VI refers to a set of biocompatibility testing requirements from the US. The result is a non-toxic bio-inert surface that is USP Class VI compliant. Theres even a silicon carbide surface thats compliant and various release agents used in the manufacture of silicone plastics are compliant.

Fda Usda Nsf51 Usp Class Vi Compliant Seals Products

Why You Need Certified Usp Class Vi Silicones Specialty Silicone Products Inc

1 25 1 1 4 Id Fda Usp Class Vi Platinum Silicone W Polyester Braid Food And Pharma Grade Flex Technologies Incorporated

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell

Double Bagged Usp Class Vi Liquid Funnels

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

What Is Usp Class Vi Testing Tbl Plastics
Dursan Passes Usp Class Vi Testing Why Is That Important
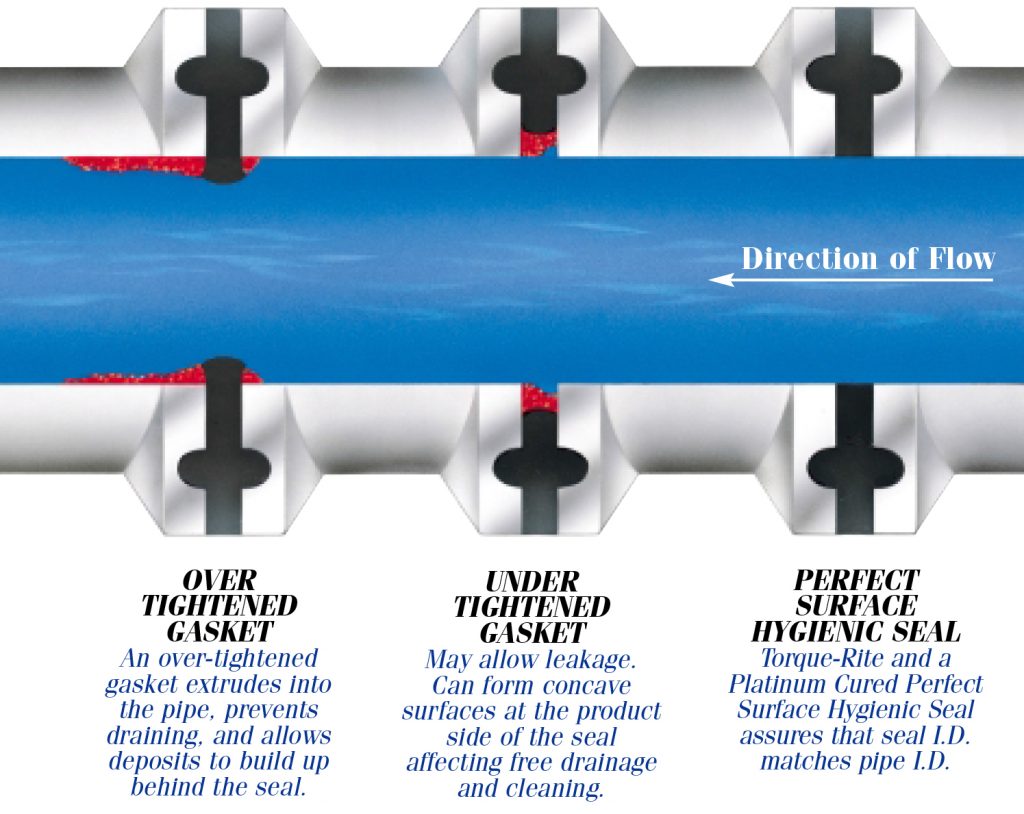
Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Standard Fda And Usp Class Vi Compliant Materials For All Types Of Hygienic Connections Repassa

Usp Class Vi What Is It And How Does It Apply To Elastomers Barnwell
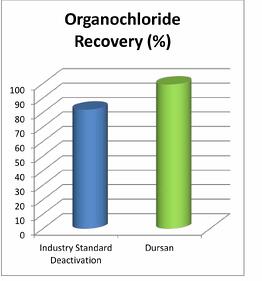
Dursan Passes Usp Class Vi Testing Why Is That Important





